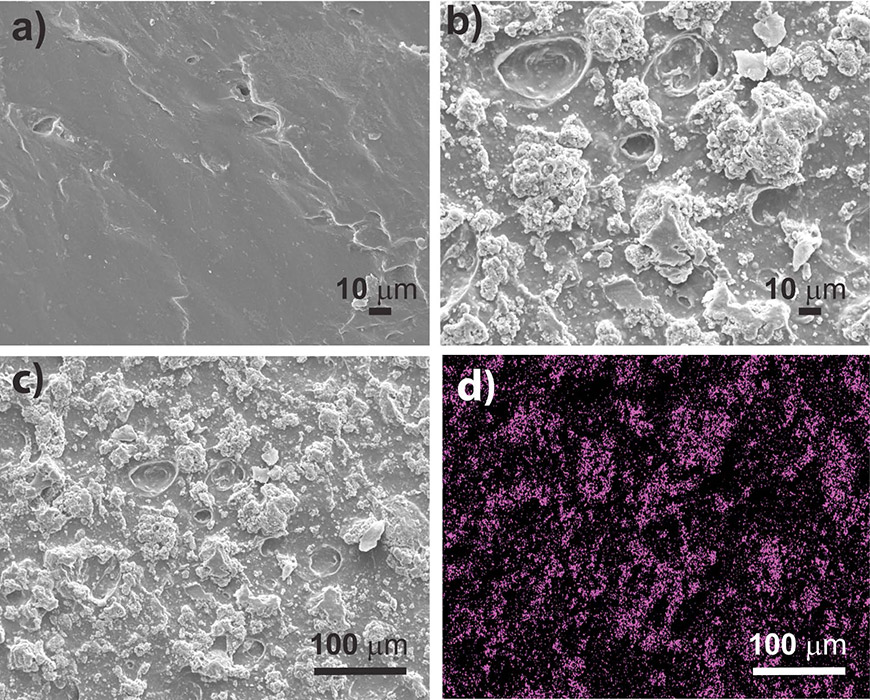
Scanning electron microscope images of (a) the chitosan film, (b) the chitosan-copper metal organic framework film at 500x magnification, (c) the chitosan-copper metal organic framework film at a higher magnification, and (d) an X-ray image of the film that shows the copper in pink.
By some estimates, bacterial strains resistant to antibiotics – so-called superbugs – will cause more deaths than cancer by 2050.
Colorado State University biomedical and chemistry researchers are using creative tactics to subvert these superbugs and their mechanisms of invasion. In particular, they’re devising new ways to keep harmful bacteria from forming sticky matrices called biofilms – and to do it without antibiotic drugs.
Researchers from the laboratory of Melissa Reynolds, associate professor of chemistry and the School of Biomedical Engineering, have created a new material that inhibits biofilm formation of the virulent superbug Pseudomonas aeruginosa. Their material, described in Advanced Functional Materials, could form the basis for a new kind of antibacterial surface that prevents infections and reduces our reliance on antibiotics.
Bella Neufeld, the first author and graduate student who led the research, explained that her passion for finding new ways to fight superbugs is motivated by how adaptive and impenetrable they are, especially when they are allowed to form biofilms.
“Biofilms are nasty once they form, and incredibly difficult to get rid of,” Neufeld said.
When bacteria attach
Many people picture bacteria and other microorganisms in their friendlier, free-floating state – like plankton swimming in a high school petri dish. But when bacteria are able to attach to a surface and form a biofilm, they become stronger and more resistant to normal drugs.
In a classic example, cystic fibrosis patients are sickened by hordes of P. aeruginosa bacteria forming a sticky film on the endothelial cells of the patients’ lungs. Once those bacteria attach, drugs won’t kill them.
Or, a wound can become infected with a bacterial biofilm, making it more difficult for that wound to heal.
Reynolds’ research group makes biocompatible devices and materials that resist infection and won’t be rejected by the body. In this most recent work, they’ve designed a material with inherent properties that keep a bacterial film from forming in the first place.
85 percent reduction
In the lab, they demonstrated an 85 percent reduction in P. aeruginosa biofilm adhesion. They conducted extensive studies showing the reusability of their film. This indicated that its antibacterial properties are driven by something inherent in the material, so its efficacy wouldn’t fade in a clinical setting.
They used a material they’ve worked with before for other antimicrobial applications, a copper-based metal-organic framework that’s stable in water. They embedded the copper metal-organic framework within a matrix of chitosan, a material derived from the polysaccharide chitin, which makes up insect wings and shrimp shells. Chitosan is already widely used as a wound dressing and hemostatic agent.
Neufeld says the new biomaterial could form new avenues for antibacterial surfaces. For example, the material could be used for a wound dressing that, instead of gauze, would be made of the chitosan matrix.
The research combined expertise in materials synthesis and biological testing. Co-authors with Neufeld and Reynolds were CSU graduate students Megan Neufeld (no relation) and Alec Lutzke; and Lawrence University undergraduate student Sarah Schweickart.